NHS patients battling Alzheimer’s will miss out on a 'miracle’ drug proven to tackle the devastating and deadly disease, health chiefs today announced.
Lecanemab was hailed as 'the beginning of the end’ of dementia last year, after studies showed it slowed the memory-robbing illness in its early stages.
And today it was approved by medicines regulator, the Medicines and Healthcare products Regulatory Agency (MHRA).
But draft guidance by the NHS spending watchdog ruled the benefits of the drug 'are too small’ to justify the estimated to be up to £1bn a year roll-out cost.
It means lecanemab will only be available to Brits who could pay around £20,000 privately every year.

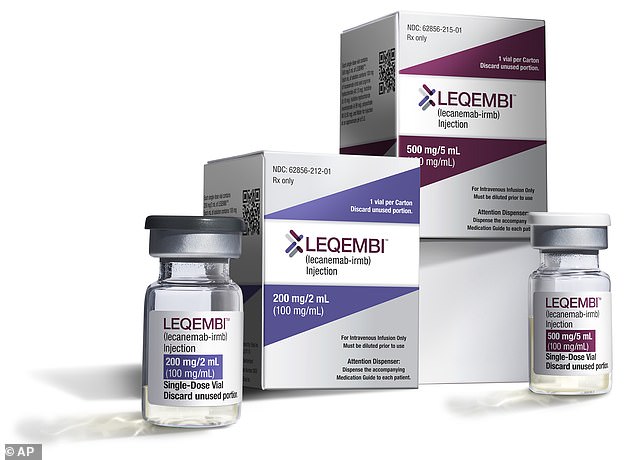
Lecanemab (pictured) has been proven to slow the progress of the memory-robbing illness in its early stages. It was today approved by medicines regulator, the Medicines and Healthcare products Regulatory Agency (MHRA)

Alzheimer’s disease is the most common cause of dementia. The disease can cause anxiety, confusion and short-term memory loss
Campaigners and charities today labelled the decision 'deeply disappointing’. However, experts also pointed to a 'lack of evidence’ over its long-term side effects.
It is estimated around 70,000 adults in England would have been eligible for treatment with lecanemab if approved for use on the health service.
The drug has been proven to slow progression of the disease by as much as 27 per cent in trials by helping to remove the build up of the harmful protein amyloid in the brains of people with early-stage Alzheimer’s.
Known as an amyloid immunotherapy, it is thought that these proteins interfere with messages sent between different parts of the brain, resulting in the memory and independence robbing symptoms.
Experts agree it could herald a new era of dementia treatment by tackling the cause rather than just alleviating symptoms.
The drug has already been given the green light in the US, China, Japan, Hong Kong, South Korea and Israel.
No price for lecanemab has been publicly announced in the UK, but in the US it costs £20,000 a year.
But it was rejected by EU medicines regulator the European Medicines Authority last month, due to concerns over side effects such as 'swelling’ and 'potential bleedings in the brain’.
It is now the first medicine to be licensed in the UK that has been shown to slow down progression of the disease.
Previously, the only available drugs for Alzheimer’s in the UK were to treat symptoms.
However, National Institute for Health and Care Excellence (NICE) chief medical officer Professor Jonathan Benger said in addition to the cost of the drug, the NHS faced a £500 bill for each patient visit to get an infusion.
Patients received the drug every two weeks for 18 months in clinical trials, and experts have said additional monitoring for side effects including brain scans mean additional costs could spiral.
A public consultation on NICE’s draft guidance will close on 20 September.
Dr Samantha Roberts, chief executive of NICE, said: 'This is a new and emerging field of medicine which will no doubt develop rapidly.

Around 900,000 Brits are currently thought to have the memory-robbing disorder. But University College London scientists estimate this will rise to 1.7million within two decades as people live longer. It marks a 40 per cent uptick on the previous forecast in 2017
’However, the reality is that the benefits this first treatment provides are just too small to justify the significant cost to the NHS.
’Our independent committee has rigorously evaluated the available evidence, including the benefit for carers but NICE must only recommend treatments that offer good value to the taxpayer.’
Meanwhile, Tara Spires-Jones, professor of the UK Dementia Research Institute at the University of Edinburgh, said the arrival of the drug marked 'a turning point’ but warned of 'dangerous side-effects’.
Speaking on BBC Radio 4’s Today programme, she said: 'It’s the first time we’ve actually been able to slow disease progression at all. So from that standpoint, it’s amazing.
’However, the treatment’s not perfect. It only slows disease progression moderately, and it comes with dangerous side-effects so patients need to be monitored very carefully, and only certain people will be able to use the drug.
’So together, it’s great news, but we have to temper our enthusiasm.’
She added: 'Some people who take this drug have brain swelling and bleeding and a few people have died from those side-effects.’
Despite these words of caution, charities and campaigners today urged the Government to 'find a solution’ to prevent Brits with dementia from missing out on the drug.
Hilary Evans-Newton, chief executive at Alzheimer’s Research UK, said: 'Today’s news is bittersweet for people affected by Alzheimer’s disease.
’It’s a remarkable achievement that science is now delivering licensed treatments that can slow down the devastating effects of Alzheimer’s, rather than just alleviating its symptoms.
’However, it’s clear our health system isn’t ready to embrace this new wave of Alzheimer’s drugs.
’It means that, as things stand, people in the early stages of the disease will be denied access to lecanemab through the NHS, and it will only be available to those who can pay privately. This is deeply disappointing.’
She added: 'Of course, like first-generation treatments for other diseases, lecanemab has modest benefits and side effects that need careful monitoring.
’It’s not a cure, but it is a real step forward — the first new dementia medicine to be licensed in more than 20 years.
’Further negotiations between NICE, Eisai and the NHS may offer a way forward.
’But the heartbreaking reality is that those who could benefit from drugs like lecanemab don’t have time to wait.
’We’ve written to the Health Secretary Wes Streeting urging him to act, and to find a solution so that people with dementia in the UK don’t continue to miss out on innovative treatments.
’There are now more than 160 trials underway testing over 125 experimental treatments for Alzheimer’s across the globe, including 30 in late-stage trials.
’Despite today’s frustrating news, it really is a matter of when, not if, [they] become available.’
Meanwhile, Fiona Carragher, chief policy and research officer at the Alzheimer’s Society, said: 'While we welcome the MHRA approval, it is disappointing that NICE has not recommended approving lecanemab for use on the NHS at this stage.
’The news that lecanemab will be restricted to certain groups of patients will also lead to uncertainty for many people with Alzheimer’s disease and their loved ones.’
Former Prime Minister and ex-president of Alzheimer’s Research UK, David Cameron, also labelled the MHRA’s decision a 'hugely positive moment’ bur cautioned 'there is still lots more to be done’.
He said: 'Today’s news that the drug will be licensed by the MHRA is another hugely positive moment.

No price for the drug has been publicly announced in the UK. In the US the treatment costs £20,000 a year. But it was rejected by EU medicines regulator the European Medicines Authority last month, due to concerns over side effects such as 'swelling’ and 'potential bleedings in the brain’
’There is still lots more to be done, but nothing breeds success like success, so I hope today’s news will trigger more research, more breakthroughs and get us closer to the point where we have what I call a „statin for the brain” — a simple pill that those at risk can take, that radically reduces the likelihood of this dreadful disease.’
Professor Sir Stephen Powis, NHS England national medical director, also said health service staff were 'looking ahead to 27 other drugs which are currently in advanced clinical trials that could be potentially approved by 2030′.
It comes as, it was revealed in April that around 5,000 Brits could get cheap blood tests to spot Alzheimer’s in efforts to revolutionise 'shocking’ NHS diagnostic rates.
Under two landmark trials, researchers from Oxford and University College London are set to use tests to detect proteins in the blood linked to the disease.
At the time, researchers said they hoped the 'groundbreaking’ blood test, which costs around £100, could speed up the process, allowing patients to get treated earlier.
The trials, which are expected to cost about £10million, are set to take place across the UK and will be carried out with people who have reported symptoms to their GP and who may be in the early stages of dementia.
A report published earlier this year by NHS England said 'timely diagnosis of dementia is vital’, adding new blood tests could soon replace lumbar punctures in diagnosing the disease.
Recent analysis by the Alzheimer’s Society estimates the overall annual cost of the disease is £42billion a year, with families bearing the brunt.
But the growing, ageing population means the charity estimates these costs – which include lost earnings of unpaid carers – are set to soar to £90billion in the next 15 years.
Around 944,000 in the UK are thought to be living with dementia, while the figure is thought to be around 7million in the US.
Alzheimer’s affects around six in 10 people with dementia.
It is thought to be caused by a build-up of amyloid and tau in the brain, which clump together and from plaques and tangles that make it harder for the brain to work properly.
Eventually, the brain struggles to cope with this damage and dementia symptoms develop.
Memory problems, thinking and reasoning difficulties and language problems are common early symptoms of the condition, which then worsen over time.
Dementia are expected to sky-rocket in the coming years, making a cheap screening tool vital to get to grips with the challenge.
Alzheimer’s Research UK analysis found 74,261 people died from dementia in 2022 compared with 69,178 a year earlier, making it the country’s biggest killer.